how to draw molecular orbital diagram of o2
Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. The molecular orbital energy diagram for O 2 predicts two unpaired electrons.

Use Mo Diagrams And The Bond Order From Them To Answer Each Of The Following Questions A Is O2 Stable Or Unstable B Is Be2 Diamagnetic Or Paramagnetic Study Com
Draw the molecular orbital diagram of O2 a.
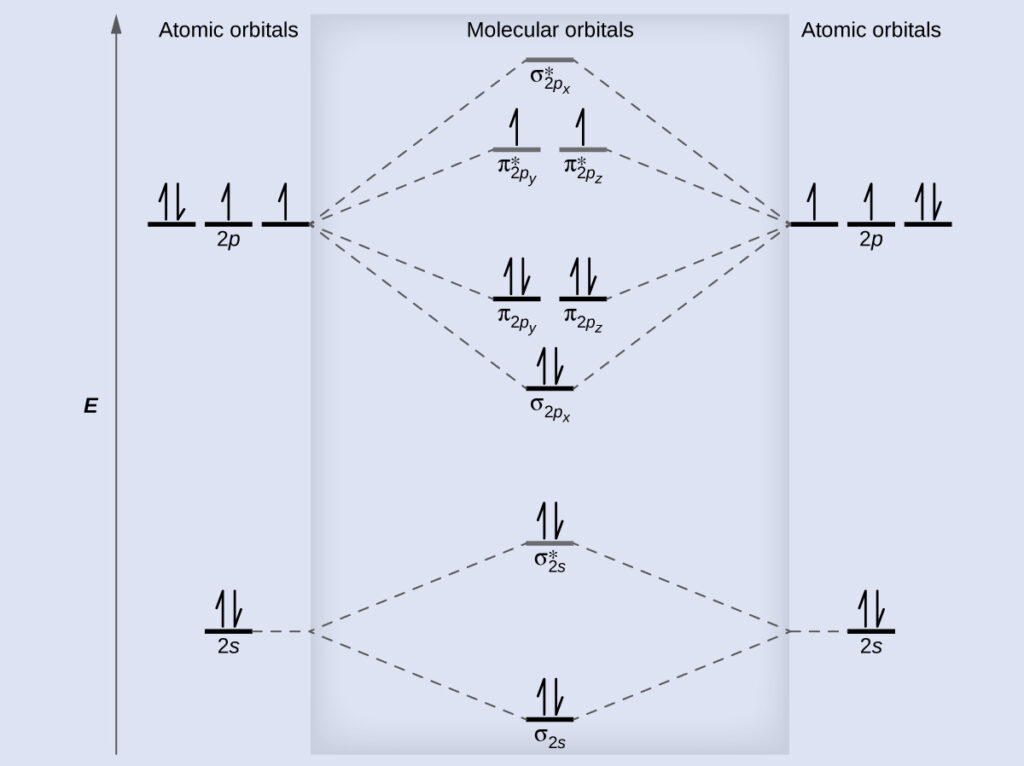
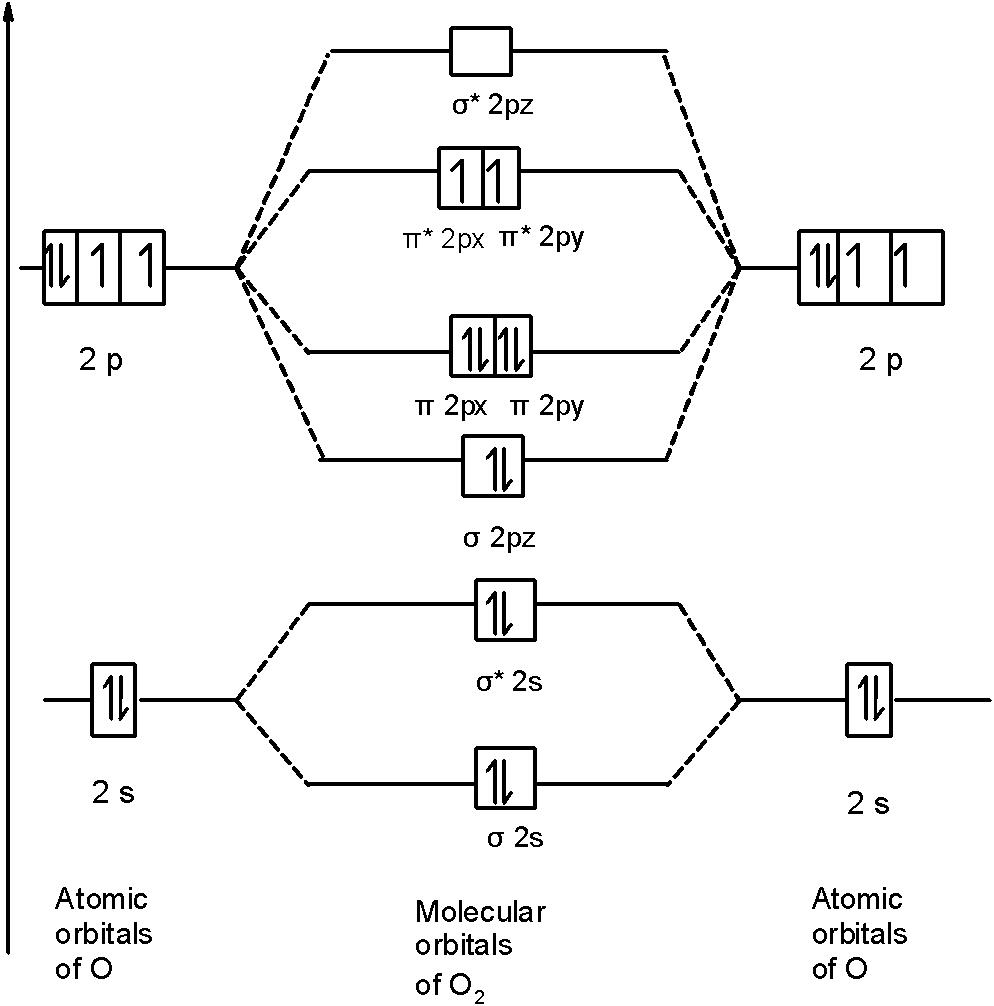
. The atomic number of Oxygen is eight and electronic configuration is 1s2 2s2 2px2 2py1 2pz1. Text O_ 2phantom rule 02em 0exfrac left 8-4right 2phantom rule 02em 0ex2 Oxygens paramagnetism is explained by the presence of two unpaired electrons in the π 2py π 2pz molecular orbitals. Na 4The two oxygen atoms in a molecule of oxygen are united through.
Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule represented as KK the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is showni Electronic configurationii Bond order. - N 2 molecules are diamagnetic with no unpaired electrons. H 1s 1 1 valence electron F He 2s 2 2p 5 7 valence electrons STEP 2.
Bond order 2 N b N a 2 8 4 2 Thus oxygen molecule has two bonds. By removing the 2 highest electrons which reside in antibonding orbitals to make O2 2 the calculation becomes 602 3. Press J to jump to the feed.
So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0. So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each. What orbital is O2s HOMO.
Compare the bond order to that seen in the Lewis structure remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a. Bond order is calculated as. Each oxygen bonds to the other with its 1s 2s and 2p orbitals.
Construct a qualitative molecular orbital diagram for chlorine Cl 2. Fill the MOs with electrons. 12 Bonding orbitals - Antibonding orbitals 128-4 2.
Depending on if it is a homonuclear case where the bonding atoms are the same or a heteronuclear case where the bonding atoms are. Each oxygen atom combines its 2s 2p z and 2p ddiagram orbitals to make three 2sp 2 hybrid orbitals. How many molecular orbitals are in O2.
How to draw the molecular orbital diagram of O2. Bonding e - antibonding e 2. So the formula to find bond order is.
Number of electrons in antibonding orbitals. Fill from the bottom up with 12 electrons total. The first major step is understanding the difference between two major theories.
8 - Drawing Molecular Orbital Diagrams. However experiments show that it is paramagnetic having tw. In normal O2 there are 6 bonding electrons and 2 antibonding electrons making the bond order 2.
F will be lower on the diagram. Bond order 1 2 Number of electrons in BMO Number of electrons in ABMO Bond order 1 2 8 2 Bond order 1 2 6 Bond order 3. Predict the bond order of Oz 10712 c.
Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. I wouldnt violate the au. The molecular orbital energy level diagram of oxygen molecule is given as follows.
Here Nb 8. Chemistry questions and answers. 2O 1s2 2s2 2px2 2py1 2pz1 à O2 2s2 2s2 2px2 π2py2 π2pz2 π2py1 π 2px1 π2px0 The valance bond theory predicts that O2 would be dimagnetic.
Do the number of AOs number of MOs. Sigma2s 2sigma2s 2sigma2p 2pi2p 4pi2p 2. Bonding Order is 2 and it is Paramagnetic.
Draw a molecular orbital diagram for ozone. Consistent with Oxygens double bond. Sorry the Sigma-1-s orbital is a little off-screen.
Rest assured its filled. Press question mark to learn the rest of the keyboard shortcuts. AO2px AO2px π2px π 2px weak sidelong overlap AO2py AO2py π2py π 2py weak sidelong overlap AO2pz AO2pz σ2pz σ 2pz strong head-on overlap Thus we take 10 atomic orbitals and generate 10 molecular orbitals in.
Valence Bond Theory and Molecular. Show the reducibleif applicable and irreducible representations for each set of group orbitals constructed and the symmetry of each orbital or group orbitals used in the diagramMultiple Bonds in. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry.
Next well see that symmetry will help us treat larger. Ie one is bond and one p bond. Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds.
Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. Which one is its LUMO. These 5 atomic orbitals combine to form 10 molecular orbitals.
Number of electrons in bonding orbitals. Molecular Orbital Diagram for Oxygen Gas O2.

Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com
Why Is The Molecular Orbital Diagram For O Different From N Quora
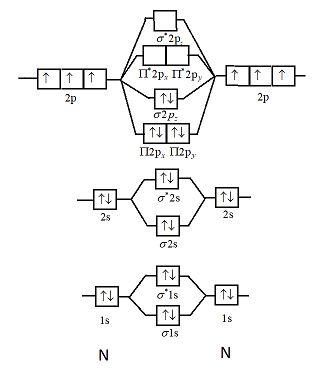
Draw The Molecular Orbital Diagram Of N2n2 N2 Write Class 11 Chemistry Cbse
What Is The Molecular Orbital Diagram For Oxygen Quora

Molecular Orbital Diagrams Bond Order And Number Of Unpaired Electrons Chem Textbook

Explain The Formation Of O2 Molecule By Molecular Orbital Theory M O T Brainly In

Orbital Energy Level Diagram At Level

8 Drawing Molecular Orbital Diagrams Flux Science

Construct Molecular Orbital Diagram And Determine Unpaired Electrons In O2 O2 Bn No Study Com

Introduction To Molecular Orbital Theory Chemistry Education Chemical Science Physical Chemistry

Draw A Molecular Orbital Diagram Of N2 Or O2 With Magnetic Class 11 Chemistry Cbse

Why Are The Bond Angles Of H20 And Nh3 104 5 And 107 5 Chemistry Lessons Chemistry Science Chemistry

Draw Molecular Orbital Diagram For O2 Molecule Brainly In
Bond Order Ofo2 O2 O2 And O22 Is In Order A O2 Langle Class 11 Chemistry Cbse

Draw Mot Diagram For B2 Molecule And Calculate Its Class 11 Chemistry Cbse

The Electron Configuration Of Oxygen Is 1s2 2s2 2p4 Science Chemistry Electron Configuration Ionization Energy

Draw Molecular Orbital Diagram Of O2 Or N2 With Magnetic Behavior And Bond Order
What S The Mot Diagram Of O2 2 Ion Quora

Draw The Molecular Orbital Energy Diagram For Oxygen Molecule O2 And Show That I It Has A Double Bond Ii It Has Paramagnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse